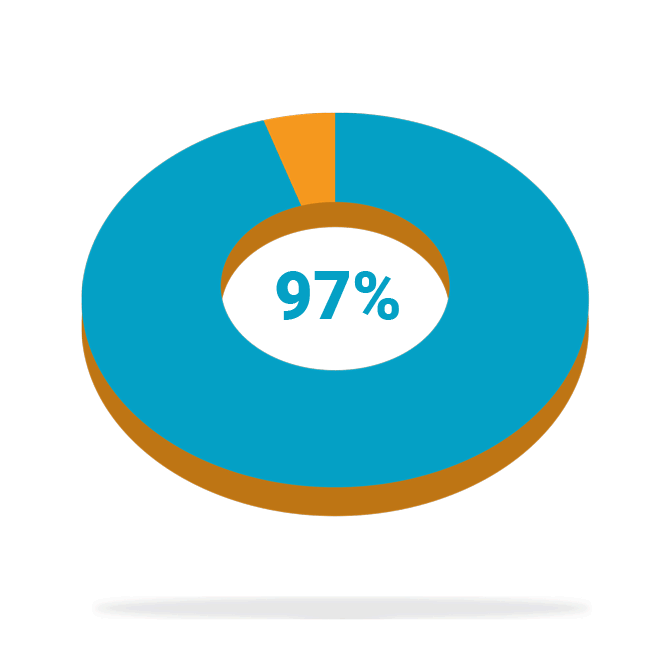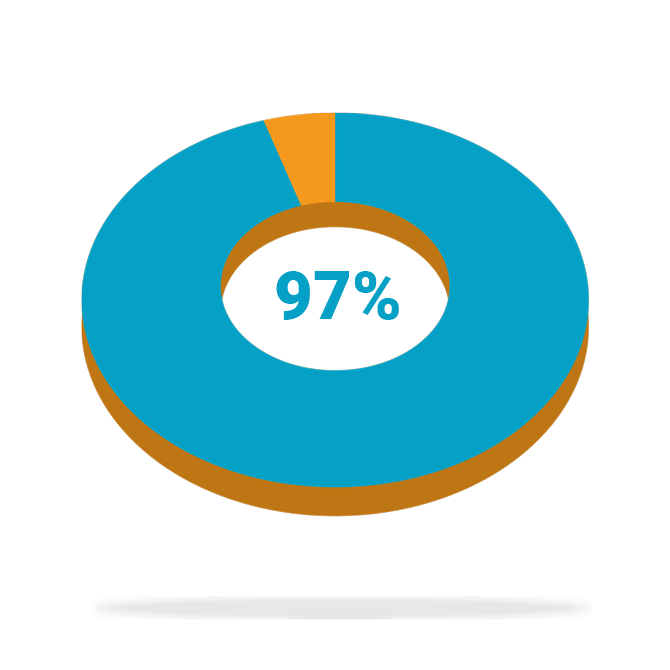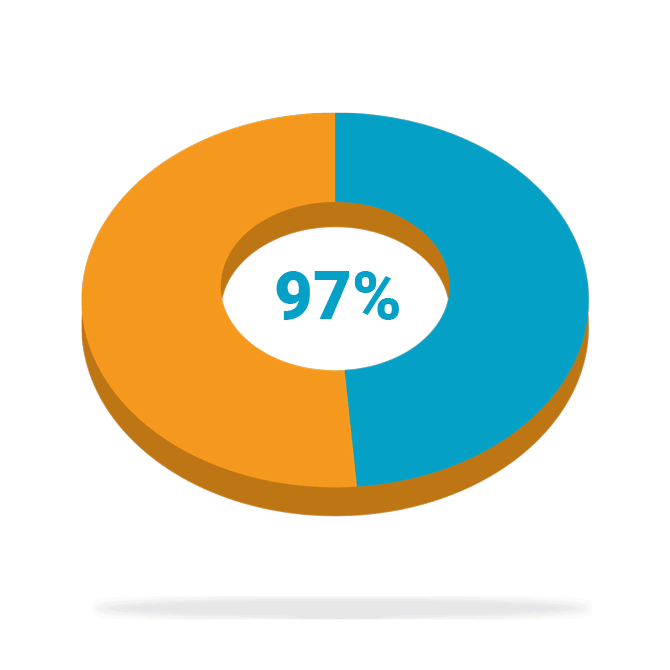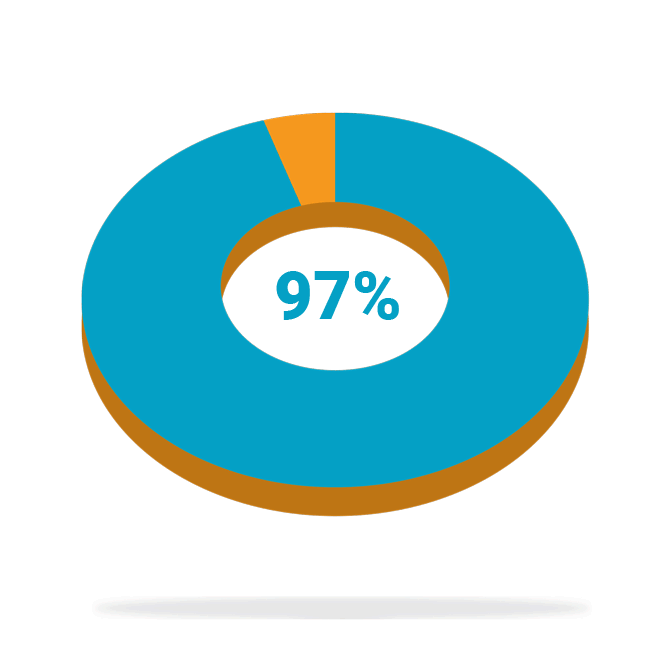
Of patients who completed the study* (88/91) were satisfied with the treatment at 24 months
High patient satisfaction
Following treatment with BlueWind Revi® in the OverActive Bladder Stimulation System Study (OASIS)—a prospective, interventional, multi-center study to evaluate the safety and efficacy of the BlueWind implantable tibial neuromodulation System for the treatment of patients diagnosed with overactive bladder (OAB)—patients reported high levels of satisfaction and saw some or much benefit in their treatment at 24 months. In addition, 87% of patients implanted with Revi experienced a ≥10 point improvement, a clinically meaningful benefit, in their Health-Related Quality of Life (HRQL) score at 24 months.
Proven efficacy, durable results
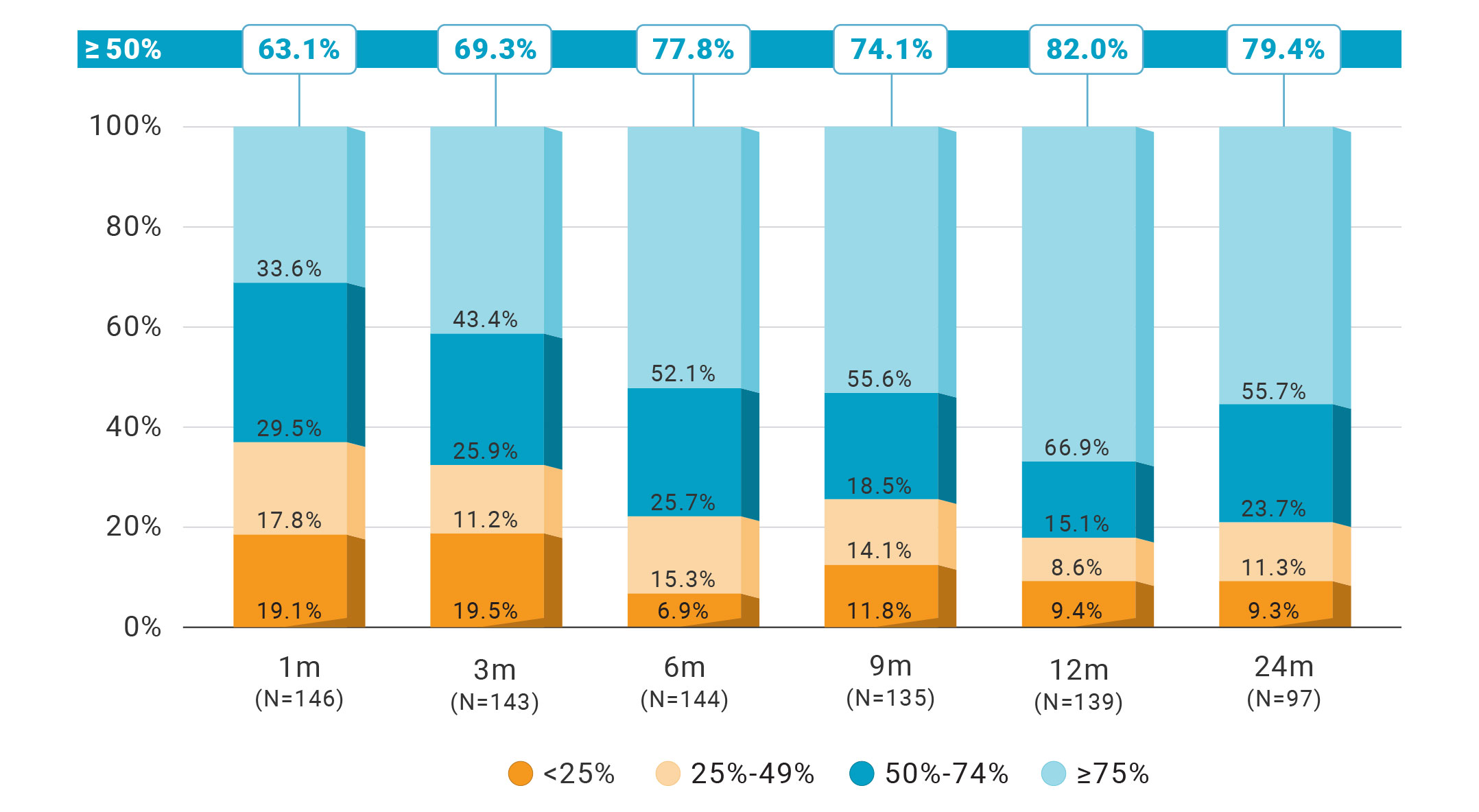
In the same study, Revi began seeing significant reductions in urge urinary incontinence (UUI) episodes as early as one month. At 24 months (N=97)*, Revi maintained efficacy and durable outcomes:1
79%
of patients had ≥ 50% reduction in UUI episodes.
56%
of patients had ≥ 75% reduction in UUI episodes.
28%
of patients were dry on a 3 day diary.

Favorable safety profile
Revi’s leadless implant design with activation by a rechargeable external wearable, combined with its reproducible surgical technique, has resulted in an exceptional safety profile that underscores its transformative benefits as a urinary incontinence device. Of the patients implanted with Revi at 24 months:1,2
- No device or procedure-related serious adverse events (SAEs)
- No surgical revision/reintervention related to battery change, lead fracture, or lead migration
- No unanticipated adverse events
Patient-centric programming improves outcomes over time
With the ability to individually tailor and optimize treatment parameters, Revi demonstrates a durable improvement in UUI episodes over time, particularly in those seeing a ≥75% improvement. By 24 months, an impressive 56% of patients saw a ≥75% improvement in their UUI due to patient-centric programming.1,2
The OASIS pivotal trial is a prospective, single-arm, open-label study conducted to evaluate the safety and efficacy of the Revi System for treating patients with urge urinary incontinence (UUI).
The OASIS trial consisted of:
- 23 leading medical centers across the U.S., U.K., and Europe.
- 151 women enrolled.
- Participants experienced an average of 4.8 UUI episodes per day, with an average of 0.9 large leaks per day, prior to treatment.
- Primary endpoint was ≥50% reduction in UUI episodes compared to baseline was the primary endpoint.
Highly regarded OASIS oversight
OASIS’ Data Safety Monitoring Board and Clinical Events Committee are comprised of some of the most highly regarded physicians in academia, ensuring the highest level of scientific rigor and ethical oversight. Their objective and independent perspective is invaluable in helping to ensure that the study results are valid, reliable, and relevant.
Data & Safety Monitoring Board (DSMB)
Clinical Event Committee (CEC)
*Completers analysis represents the patients with available data at 24 months.
For further information on the OASIS study and other on-going clinical studies, please visit clinicaltrials.gov (OASIS NCT03596671)
References
1. Amundsen, C. et al. 2 Year Results: Revi® Therapy, Implanted Neuromodulator for Urgency Incontinence. AUGS Oct. 2024
2. Sutherland, S et. al. OASIS Pivotal Trail to Evaluate the Safety and Efficacy of the Renova iStim System and for the Treatment of Women with OAB. Jurol. 2022; 207(S5):e1043.
3. Dorsthorst MJ, Digesu GA, Tailor V, Gore M, van Kerrebroeck PE, van Breda HMK, Elneil S, Heesakkers J. 3-year follow-up of a new implantable tibial nerve stimulator for the treatment of Overactive Bladder Syndrome. Jurol Vol. 204, 545-550, September 202. https://doi.org/10.1097/JU.0000000000001024








