Is Revi right for me? Talk to your doctor
Your primary care provider is typically the first medical professional you discuss your bladder symptoms with. They are often the providers who diagnose and provide initial treatment for those with urge urinary incontinence.
If lifestyle changes haven’t helped and medications aren’t for you, it may be time to speak with a Revi trained urologist or urogynecologist who can help determine if Revi may be the solution you’re looking for.

Prepare for Your Appointment
If this is your first time seeing a urologist or urogynecologist, this is your opportunity to talk about your bladder symptoms and the treatments you’ve tried with a specialist. You might also be asked to prepare for your appointment by providing a bladder diary. It may be helpful to write down specific questions you have. Download a discussion guide to help you plan for the conversation with your healthcare provider.

Insurance coverage
If you and your healthcare provider agree that Revi is the best option for you, if required, they may submit for prior authorization with your insurance to better understand your coverage benefits. Since Revi is a new type of treatment, this process may take a little longer than usual. BlueWind has partnered with a prior authorization support company to assist with this process and keep you and your doctor informed of the status of your request.
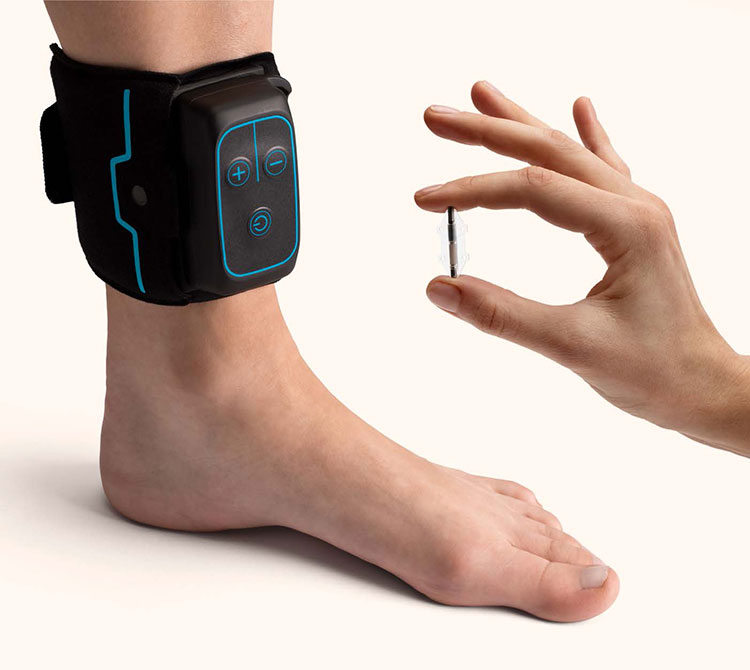
Get ready for Revi
The Revi Implant is placed during a single out-patient procedure. Because it is miniature in size, Revi can be implanted under local anesthesia via a small incision in your ankle region. You’ll be able to walk out of the hospital or outpatient clinic after the procedure is complete. Your doctor will allow for the incision site to heal for several weeks before turning on and personalizing your Revi therapy.

“I’m amazed when I see how a single procedure has the possibility of transforming someone’s life who has been suffering from urge urinary incontinence."
— Kristin, Surgical Procedure Specialist
Does the Revi Procedure have side effects?
Revi is proven to be safe and effective without the side effects associated with drugs. In the recent OASIS study, Revi demonstrated a favorable safety profile with no serious complications* related to the device or procedure at 24 months.1
What does it feel like when you’re using Revi?
When you’re conducting a Revi treatment, you will feel a mild tingling in the foot. When you feel this, it’s normal and means that the therapy is working correctly.
How often do I use Revi?
You will work with your provider to determine the number of treatments you’ll need a day. You can give yourself treatment sessions when and where it is most convenient for you by placing your wearable around your ankle to deliver therapy.

Personalizing Revi
This is the day you’ve been waiting for! You’ll meet with a member of your care team, who will program Revi and personalize the settings, so they are right for you. They’ll show you how to use the Revi System and answer any questions you might have. This team will be available throughout your journey to ensure you’re getting the best results possible from Revi.
*Some women (11.9%) had mild to moderate issues, including numbness after the procedure, pain, swelling, skin rash, wound infections, and delayed healing.(2)
For further information on the OASIS study and other on-going clinical studies, please visit clinicaltrials.gov (OASIS NCT03596671)
(link clinicaltrials.gov (OASIS NCT03596671) : https://classic.clinicaltrials.gov/ct2/show/NCT03596671)
References:
1. Heesakkers JPFA, Toozs-Hobson P, Sutherland SE, Digesu A, Amundsen CL, McCrery RJ, et al. Two-Year Efficacy and Safety Outcomes of the Pivotal OASIS Study Using the Revi System for Treatment of Urgency Urinary Incontinence. J Urol 2025 Mar 1;213(3):323–32. https://doi.org/10.1097/JU.0000000000004328
2. Revi Surgical Technique Guide May 2024