

Awarded Best New Technology Solution – Therapeutics at the 2025 MedTech Breakthrough Awards.
Transforming neuromodulation for urge urinary incontinence
BlueWind Revi® was crafted with the patient and the physicians who treat urge urinary incontinence (UUI) in mind. The advanced design of our implant for urinary incontinence allows for the device to be miniature in size without the need for replacement surgeries for battery change, lead migration, or lead fracture. Revi’s innovative external wearable technology powers the implant for a patient-centric approach to therapy, allowing for stimulation sessions based on the patients’ needs, without concern for energy consumption, while giving you the flexibility to ensure the best possible outcome.
In the OverActive Bladder Stimulation Study (OASIS) Completers analysis* (N=97) at 24 months, Revi demonstrated:¹
97% of patients were satisfied with the treatment.
79% of patients had a ≥ 50% reduction in UUI episodes.
28% of patients were dry on a 3 day diary.
Revi System’s streamlined components

Revi Implant is activated by a battery-operated external Wearable allowing it to be miniature in size at only 3 cm in length and 3 mm in diameter. This tibial implant delivers reliable and long-lasting performance in a compact form factor.

Revi Wearable is only 7.8 oz, delivering therapy at the patient’s convenience, when, where, and how often they want. Multiple treatment programs allow patients to adjust programs as needed.
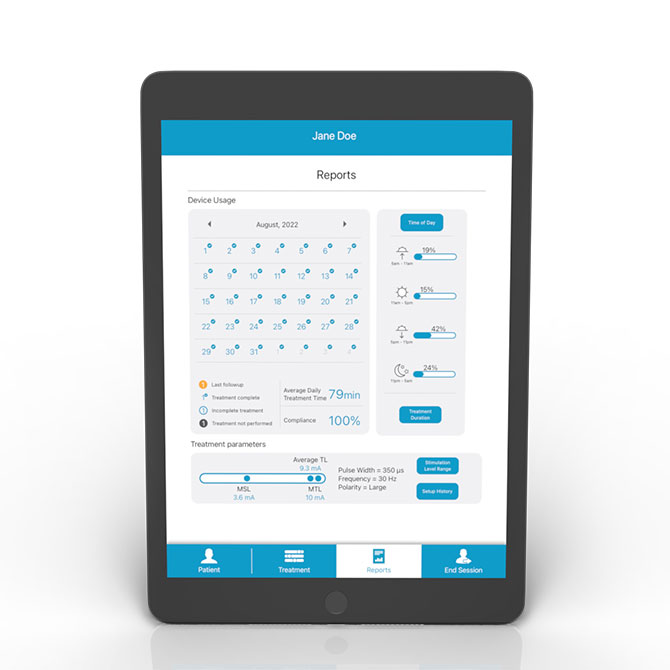
Revi Clinician Programmer allows for the customization of therapy based on a patient’s response. Includes adjustment of frequency, pulse width, polarity, and amplitude.

Hub wirelessly communicates therapy data from the Wearable, allowing clinicians visibility into device usage and status to better support and optimize patient therapy.
The Revi Implant is MR conditional and is safe for 1.5T and 3T full body MR scans.

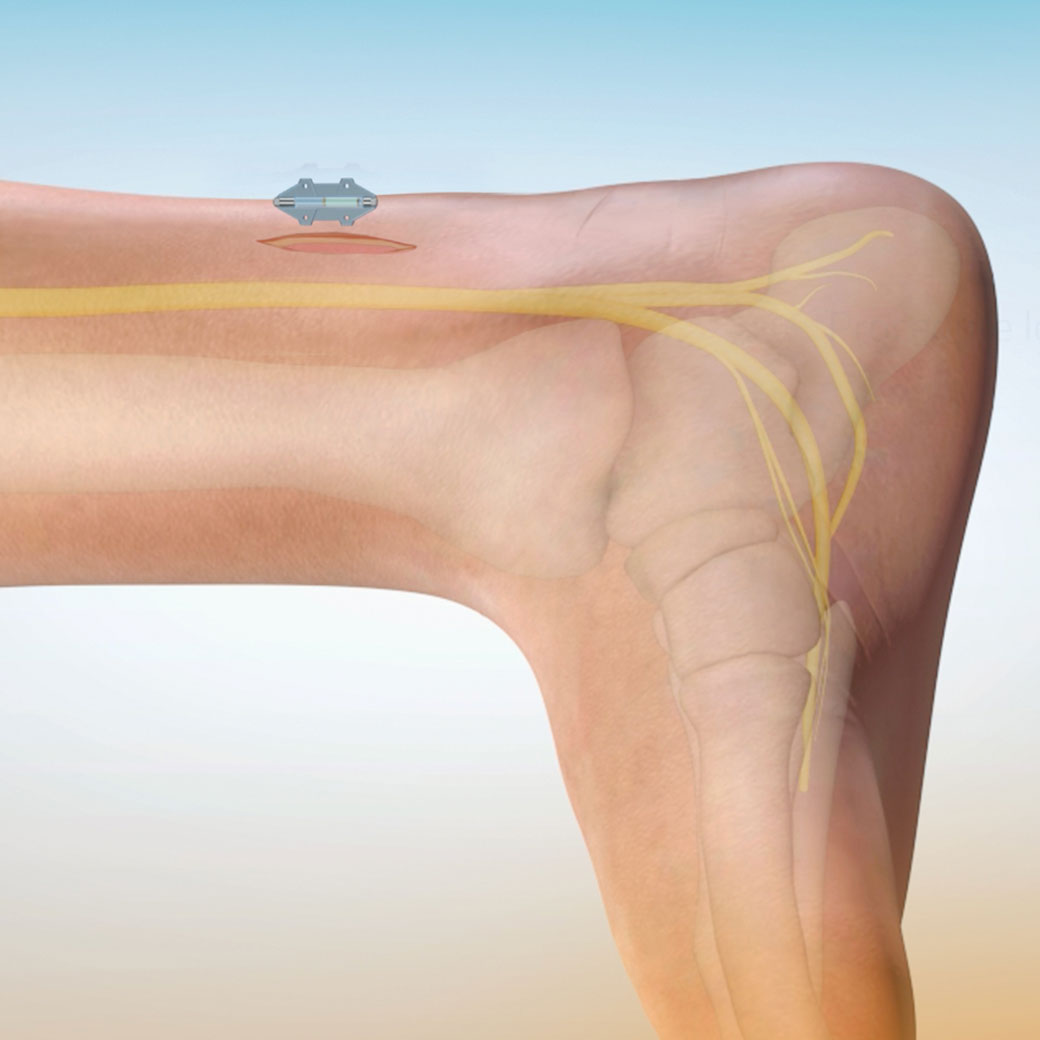

Thoughtful design, miniaturized
This patented and novel miniature implant for urinary incontinence harnesses the power of modern wireless technology without the need for a bulky implant. It enables the battery to be externalized in the Wearable allowing for a more tailored treatment experience without concern for battery longevity.

Single procedure, proven results
The unique implant placement and system design provides an alternative solution for patients seeking treatment:
- Minimally Invasive. Miniature device is implanted in a single predictable procedure under local anesthesia.
- Confident placement. Subfascial placement allows for direct visualization of the tibial nerve. Intraoperative testing and suture fixation provides for more predictable outcomes.
- Customized therapy. Optimized treatment based on patient response. Dashboard allows provider visibility into device usage.
*Completers analysis represents the patients with available data at 24 months. Satisfaction results are from 91 patients.
For further information on the OASIS study and other on-going clinical studies, please visit clinicaltrials.gov (OASIS NCT03596671)
References
1. Amundsen, C. et al. 2 Year Results: Revi® Therapy, Implanted Neuromodulator for Urgency Incontinence. AUGS Oct. 2024


